STEM CELL ENGINEERING
Research Interests
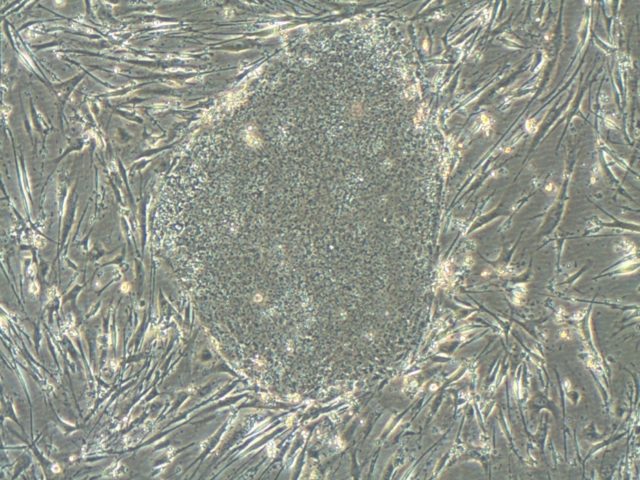
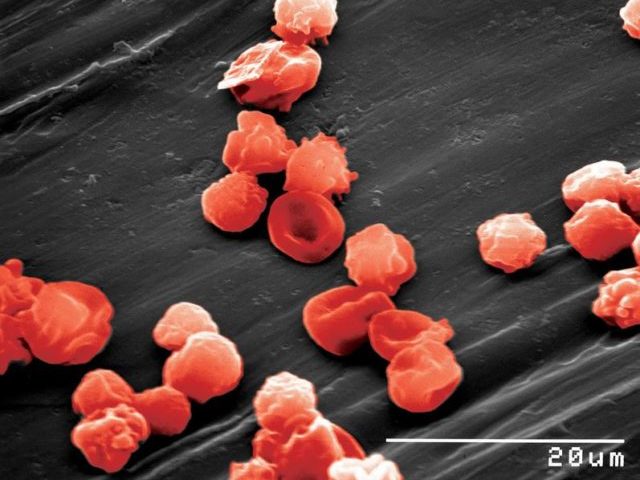
Somatic cells can be reprogrammed to a pluripotent embryonic stem cell-like state (induced pluripotent stem (iPS) cells) by combinations of the transcription factors OCT4, SOX2, NANOG, LIN28, KLF4 and cMYC (Takahashi et al., 2006, 2007; Yu et al., 2007; Park et al., 2007). The initial studies were underdone inducing pluripotency in mouse and human fibroblasts. We were able to demonstrate, that mouse and human neural stem cells which express SOX2, KLF4 and cMYC, can be reprogrammed to iPS cells by retroviral expression of OCT4 (Kim, Zaehres et al., 2008, 2009). Afterwards, we have comparatively evaluated the hematopoietic and erythroid as well as neural differentiation potential of human cord blood and neural stem cell derived iPS cells (Hargus et al., 2014, Dorn et al., 2015).
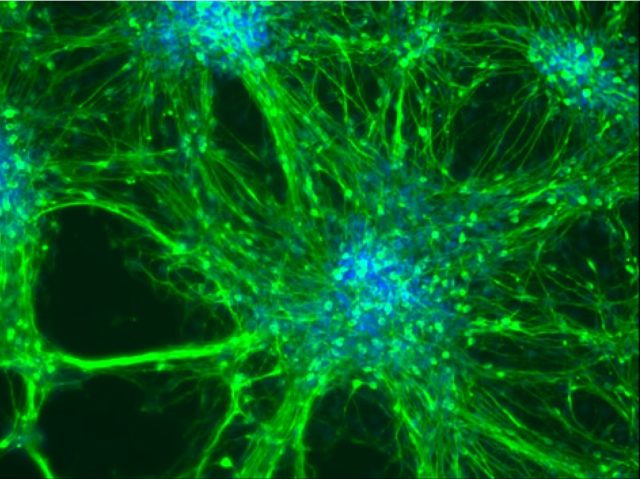
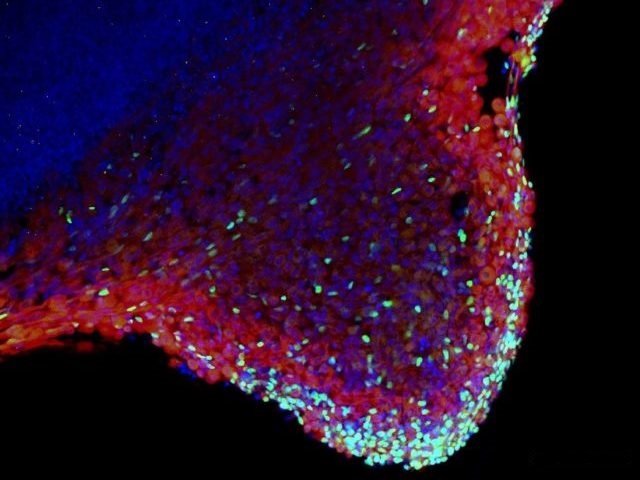
Patient-derived induced pluripotent stem cells and their differentiation derivatives hold great promise as novel in vitro model systems to study human pathogenesis. We are currently focusing to model neural (Frontotemporal Dementia) and skeletal muscle (Duchenne, LGMD) diseases with human iPS cells and CRISPR/Cas9 genome editing. Recently, we have developed a human skeletal muscle organoid differentiation system, which recapitulates myogenesis (Mavrommatis et al., 2020).
We are further developing our iSTEM (Molecular and Developmental Stem Cell Biology) master program since its inception in 2010 by incorporating state-of-the-art topics of stem cell research in the teaching modules.
Contact
PD Dr. Holm Zähres
Ruhr-University Bochum
Institute of Anatomy
Department of Anatomy and Molecular Embryology
44801 Bochum, NRW, Germany
- Tel: +49 (0)234 32 25714
Selected Publications
Mavrommatis, L., Jeong, HW., Gomez-Giro, G., Stehling, M., Kienitz, MC., Psathaki, OE., Bixel, MG., Morosan-Puopolo, G., Gerovska, D., Araúzo-Bravo, MJ., Schwamborn, JC., Schöler, HR., Adams, RH., Vorgerd, M., Brand-Saberi, B., Zaehres, H. (2020) Human skeletal muscle organoids model fetal myogenesis and sustain uncommitted PAX7 myogenic progenitors. bioRxiv 2020.09.14.295832
Hallmann A.L., Arauzo-Bravo M.J., Mavrommatis L., Ehrlich M., Röpke A., Brockhaus J., Missler M., Sterneckert J., Schöler H.R., Kuhlmann T., Zaehres H*., Hargus G*. (2017) Astrocyte pathology in a human neural stem cell model of frontotemporal dementia caused by mutant TAU protein. Scientific Reports 7, 42991
Dorn, I., Klich, K., Arauzo-Bravo, M.J., Radstaak, M., Santourlidis, S., Ghanjati, F., Radke, T., Psathaki, O.E., Hargus, G., Kramer, J., Einhaus, M., Kim, J.B., Kögler, G., Wernet, P., Schöler, H.R., Schlenke, P., Zaehres, H. (2015) Erythroid differentiation of human induced pluripotent stem cells is independent of donor cell type of origin. Haematologica 100, 32-41
Hargus, G., Ehrlich, M., Arauzo-Bravo, M.J., Hemmer, K., Hallman, A.L., Reinhardt, P., Kim, K.P., Adachi, K., Santourlidis, S., Ghanjati, F., Fauser, M., Ossig, C., Storch, A., Kim, J.B., Schwamborn, J.C., Sterneckert, J., Schöler, H.R., Kuhlmann, T., Zaehres, H. (2014) Origin dependent neural cell identities in differentiated human induced pluripotent stem cells in vitro and after transplantation into the rodent brain. Cell Reports 8, 1697-703
Kim, J.B., Greber, B., Arauzo-Bravo, M.J., Meyer, J., Park, K.I., Zaehres, H., Schöler, H.R. (2009) Direct reprogramming of human neural stem cells by OCT4. Nature 461, 649-653
Kim, J.B.*, Zaehres, H.*, Wu, G., Gentile, L., Ko, K., Sebastiano, V., Arauzo-Bravo, M.J., Ruau, D., Han, D.W., Zenke, M., Schöler, H.R. (2008) Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454, 646-650
Zaehres, H., Lensch, M.W., Daheron, L., Stewart, S.A., Itskovitz-Eldor, J., Daley, G.Q. (2005) High-efficiency RNA interference in human embryonic stem cells. Stem Cells 23, 299-305


